Datasheet 搜索 > 变容二极管 > Infineon(英飞凌) > BB639E7904HTSA1 数据手册 > BB639E7904HTSA1 开发手册 5/18 页

 器件3D模型
器件3D模型¥ 0.501
BB639E7904HTSA1 开发手册 - Infineon(英飞凌)
制造商:
Infineon(英飞凌)
分类:
变容二极管
封装:
SOD-323-2
描述:
Infineon BB639E7904HTSA1 30V 36pF 变容管, 最小调谐比: 13.5 VHF, 2引脚 SOD-323封装, 使用于调谐器
Pictures:
3D模型
符号图
焊盘图
引脚图
产品图
BB639E7904HTSA1数据手册
Page:
of 18 Go
若手册格式错乱,请下载阅览PDF原文件
BB-IND 11184 Diphtheria Antitoxin (DAT) Protocol CDC IRB #4167 Version 7.0
Page 4 September 21, 2016
continued to increase with more prolonged intervals, reaching 29.9% in those treated 7 or more days after
onset. Current thinking is that toxin fixes to susceptible cells early in disease and fixed toxin is not
neutralized by antitoxin.
4
The management of a patient with suspected diphtheria includes:
1) Administration of DAT as soon as possible after testing for hypersensitivity to horse serum; early
administration of DAT is critical for survival.
5
2) Establishing the diagnosis through appropriate bacterial cultures;
3) Administration of antibiotics; and
4) Appropriate supportive care including special attention to maintaining an adequate airway in the
presence of laryngeal or extensive pharyngeal membranes and to careful monitoring for cardiac
rhythm disturbances or other manifestations of myocarditis.
Diphtheria is currently a rare disease in the United States. The last cultured-confirmed cases of diphtheria
with toxigenic C. diphtheriae was reported in 1999 and 3 cases of diphtheria-like illnesses caused by
toxigenic C. ulcerans and 5 probable cases of diphtheria were reported from January 2000 through
December 2015. A case of exudative pharyngitis with a positive culture for nontoxigenic C. diphtheriae
was reported in 2014. In 2003, a fatal probable case of respiratory diphtheria was imported from Haiti, a
country that is endemic for the disease.
6
Access to DAT is essential to ensure urgent and effective
treatment of all cases of suspected diphtheria. There is evidence that toxigenic strains of diphtheria
continue to circulate in at least limited areas of the United States.
7
3.0 PRODUCT INFORMATION
With the discontinued manufacturing of a U.S. licensed DAT product by Connaught in 1997, access to
DAT product in the U.S. has been through CDC under an IND program. A DAT product manufactured by
the Instituto Butantan in São Paulo, Brazil is provided for use in the United States under this IND
program. This product is similar to the previously licensed DAT.
DAT manufactured by Instituto Butantan is a sterile, transparent (clear) serum solution supplied in 10 mL
ampoules containing 10,000 IU each. The composition of DAT is summarized in Table 1. DAT must be
stored in the refrigerator at 2 - 8°C (36 – 46°F). DO NOT FREEZE. Once an ampoule is opened, the DAT
serum solution should be used immediately.
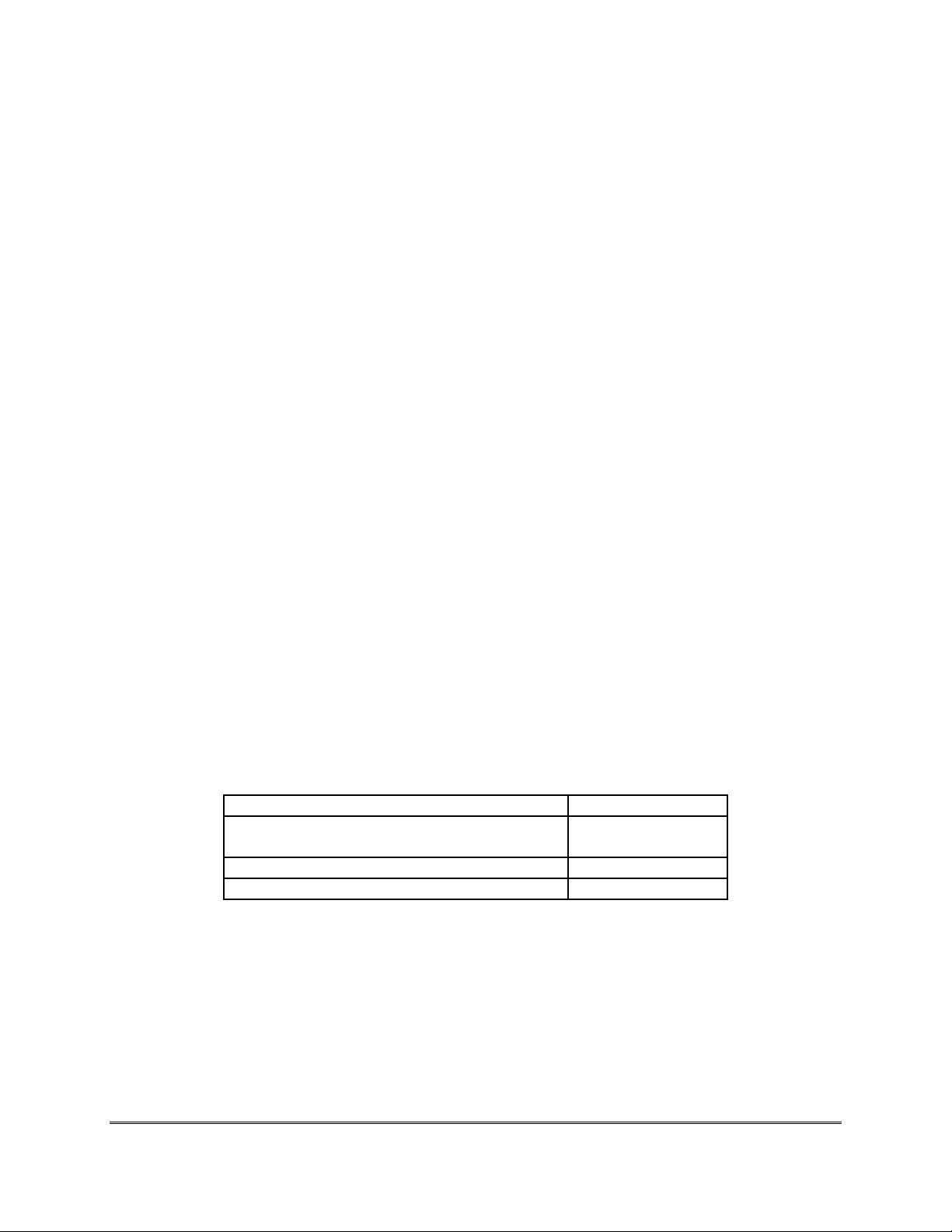
Table 1. DAT Composition – Each 10 mL Ampoule
Ingredient
Content
Diphtheria Antitoxin (DAT) immunoglobulin
antigen-binding fragments
10,000 IU
Phenol
35 mg (maximum)
0.85% physiological solution
10 mL
4.0 PROGRAM DESCRIPTION
This is a program to allow DAT treatment for patients with suspected diphtheria infection. Physicians
requesting diphtheria antitoxin should contact the CDC’s Emergency Operations Center (EOC) at 770-
488-7100. The CDC diphtheria duty officer will release DAT if, following the discussion, it is the
decision of the treating physician to request and administer DAT. The treating physician may decide
against using DAT after it is released. In requesting and obtaining DAT, the treating physician is agreeing
to serve as a site investigator under the IND and acknowledging to comply with FDA IND regulations
(please refer to Section 7.0 of this protocol).
器件 Datasheet 文档搜索
AiEMA 数据库涵盖高达 72,405,303 个元件的数据手册,每天更新 5,000 多个 PDF 文件






